Current Studies
Take a look below at the studies we have right now. Reach out to us if you are interested in joining a study!
Combination Therapy to Improve Walking Recovery after SCI (BO2ST)

Age: 18-70
Time since SCI: at least 1 year after injury
Level of SCI: C2-L2, AIS C-D (can walk 10 meters without physical assist of another person)
Screening (1 hr.): Confirmation of study eligibility
Study Intervention (20 visits over 4 weeks, 1-3 hrs.): This study is randomized, that means participants are assigned to different treatment groups and each participant has an equal chance of being assigned to any group. Eligible participants will be randomized into one of three groups, all have at least one active intervention:
- Acute intermittent hypoxia (AIH) prior to walking practice with stimulation (WALK+tSTIM)
- Acute intermittent hypoxia (AIH) prior to walking practice without stimulation (WALK+tSHAM)
- Room air (SHAM) prior to walking practice with stimulation (WALK+tSTIM)
Follow-Up (3 visits over 2 months, 2 hrs.): Assessment by a physical therapist at 1 week, 1month and 2 months post-intervention.
Example Schedule:
| Monday | Tuesday | Wednesday | Thursday | Friday |
| Screening Visit | Baseline Visit | |||
| Walking Practice | Walking Practice | Walking Practice | Walking Practice | |
| Walking Practice | Walking Practice | Walking Practice | Walking Practice | Assessment |
| Intervention | Intervention | Intervention | Intervention | Assessment |
| Intervention | Intervention | Intervention | Intervention | Assessment |
| 1-Week Follow-Up | ||||
| 4-Week Follow-Up | ||||
| 8-Week Follow-Up |
Remuneration: $40 for each completed visit, up to $300 reimbursement to help defray transportation costs
If you would like to learn more about this research study, or other ongoing research studies, please click here.
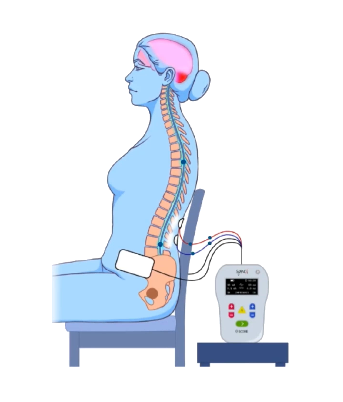
SCONE INCONTINENCE Clinical Study - Spinal Cord Stimulation Study for Adults with Neurogenic Lower Urinary Tract Dysfunction (NLUTD)
Age: 18-70 if male; 18-75 if female
Time since problem was diagnosed: 1 yr+ with Chronic Urinary Tract Dysfunction
Diagnosis: Spinal Cord Injury (SCI), Multiple Sclerosis (MS), or Stroke
If you:
- Have more than 2-4 incontinence episodes OR urinate/catheterize more than 8-10 times per day
- Do not rely on indwelling catheter
- Have not had bladder Botox in last 9 months
You might be an eligible participant in this study.
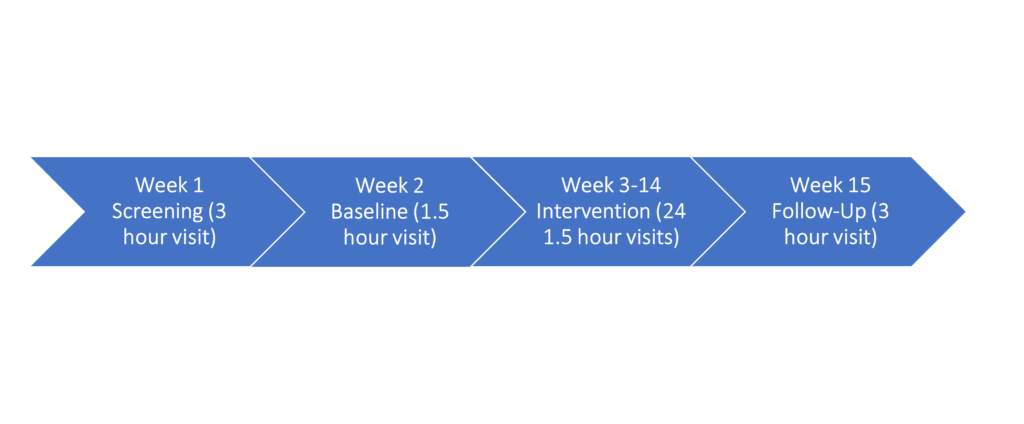
Screening and Baseline (1-2 visits): Initial information with a licensed clinician
Study Intervention (24 treatment visits): Non-invasive electric stimulation is provided through electrodes on the skin along the spine to stimulate the spinal cord
Follow-Up (1 visit): Assessment by a licensed physical therapist at 1 week post-intervention
Remuneration: $50 for each completed visit, up to $1350 total
If you would like to learn more about this research study, or other ongoing research studies, please click here.
ASPIRE - Clinical Study for Upper Limb Paralysis due to Spinal Cord Injury

Age: 22+
Time since Injury: 1 yr+
Level of SCI Injury: C2-T2 with limited function of upper extremities (Motor Score for each arm 5-20)
If you:
- Have a BMI of <40
- Do not have an active implanted medical device for electrical stimulation
- Have had no Botox in your upper limbs during the last 3 months
- Are not pregnant
You might be an eligible participant in this study and take part in developing of a new type of neuromodulation technology to complement rehabilitation programs for individuals with chronic spinal cord injury.
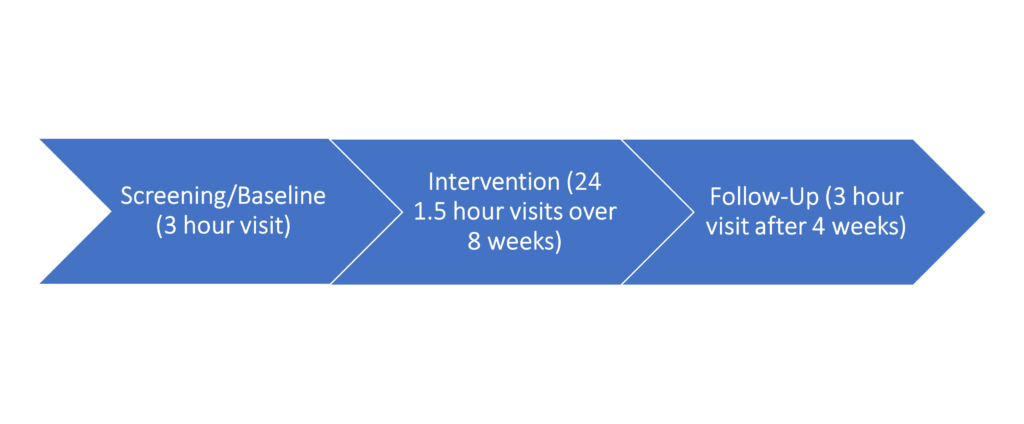
Screening (1 baseline visit, about 3 hours): Initial information with a licensed clinician
Study Intervention (24 visits over 8 weeks, 3 1.5 hour visits each week): All subjects will receive 3 weekly sessions of 1-hour upper limb rehabilitation with a licensed PT/OT for the duration of the 8 weeks of therapy.
Follow-Up (1 visit, about 3 hours): Assessment by a licensed physical therapist at 4 weeks post-intervention
Remuneration: $50 for each treatment visit (week 1-8), $100 for each assessment visit (week 4 & 8), $200 for follow-up visit (week 12), up to $1600 total
If you would like to learn more about this study, or other ongoing research studies, please click here.
Combinatorial Treatment of Acute Intermittent Hypoxia and Transcutaneous Electrical Spinal Cord Stimulation to Improve Hand Function in People with Cervical SCI

Age: 18-65
Time since Injury: 1 yr+
Level of Injury: C3-C7
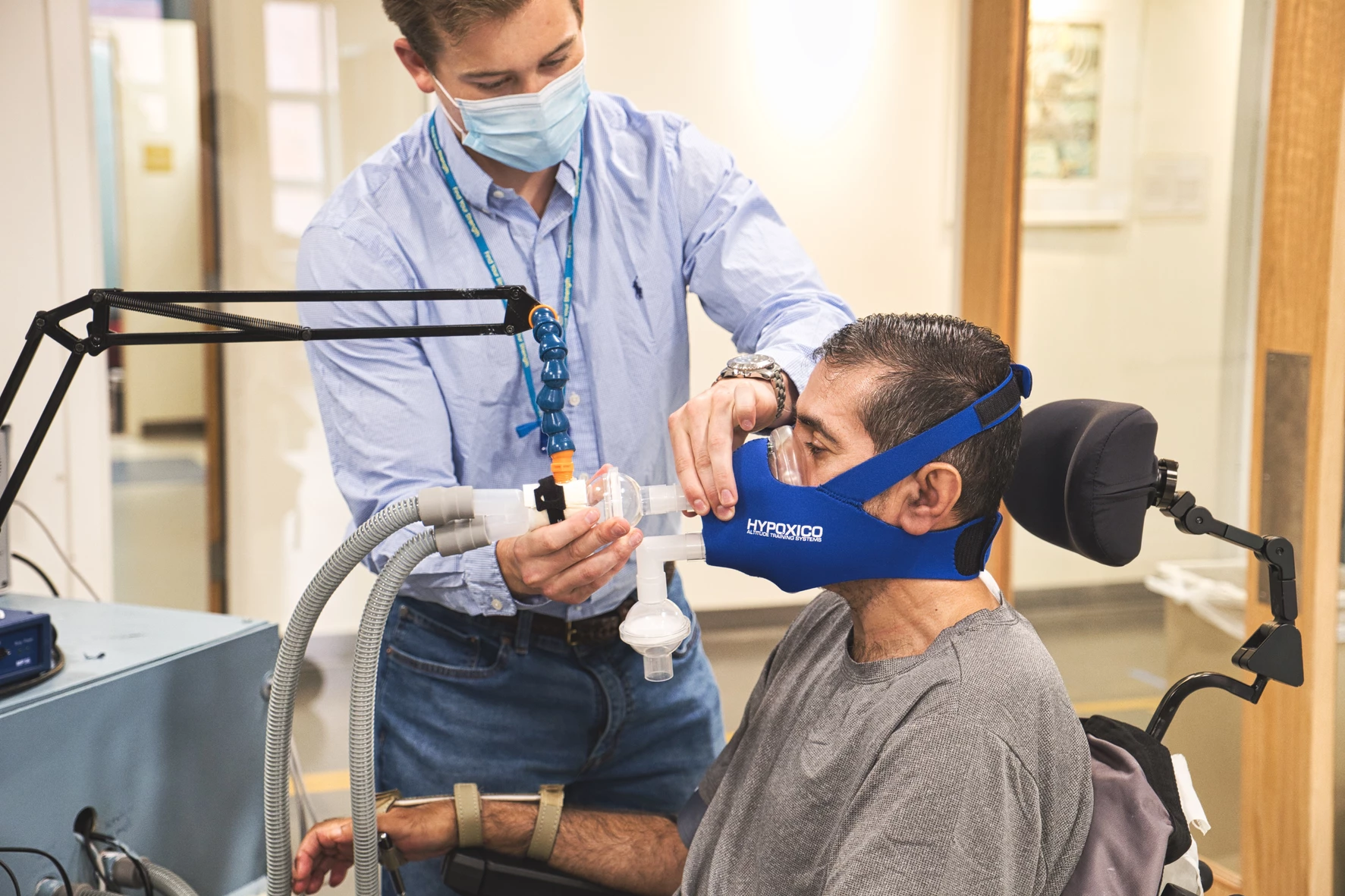
Screening: Initial information with a licensed clinician
Study Intervention (2 intervals, 15 2-3 hour visits each): Experimental breathing sessions and transcutaneous electrical spinal stimulation with upper extremity task practice over the course of three weeks
Follow-Up (2 visits, 2 hours each): Assessment by a licensed physical therapist at 1 week post-intervention
Spinal Cord Injury (SCI) Biorepository
For this study, you will be asked to provide a singular collection of saliva. While participating in this study will not directly benefit you, you will be helping us to improve precision of care in spinal cord injury rehabilitation. Genetic testing has an important role in personalized medicine because it helps researchers identify human differences relevant to the treatment of a condition. We are studying important genetic targets that may influence how, why, and when clinicians prescribe treatments.